We talk about oxidation all the time with our outdoor equipment. Yet, people don’t really understand oxidation in a way that makes sense to them. Yes, you can read any number of articles on the subject and they will explain the chemical basis of oxidation but they don’t really get down to the heart of it. We home theater types really just want to understand why oxidation can be a bad thing, and why it can be a good thing. So, I thought I’d take a try at explaining it from a home theater point of view.
First the fancy chemistry
The first thing you need to remember is that there are substances called “elements.” An element is something that has a unique, identifiable quality that makes it different from something else. That “something” is its atomic structure. An atom of iron is different from an atom of aluminum. But, if you break that atom apart, you’ll find both atoms are made up of the same stuff.
When we talk about atoms, we also talk about compounds. It’s hard for you to know the difference between atoms and compounds just with the naked eye. A compounds is a mix of two or more different kinds of atoms. You can convince those atoms to separate using chemical reactions. In order to convince an atom to separate into its parts, you need a lot more than chemistry. That’s the big difference.
Understanding oxygen
There are basically two types of elements. Metals join with non-metals. You can’t really join a metal with another metal, and you can’t join a nonmetal with another metal. Every stable molecule is a combination of at least one metal, plus at least one non-metal. Sometimes two metals will join together with a nonmetal.
Oxygen is one of the most common nonmetals on the planet and it combines easily with the metals we have here on earth. Good thing, too, because oxygen is pretty important to life as we know it. Hydrogen combines with oxygen to make water. Carbon and hydrogen combine with oxygen to make sugar, which is the basic power source for our bodies.
But oxygen’s ability to combine with other elements can also cause problems, if we don’t want those elements to combine.
Which takes us to oxidation.

Oxidation is the term for any metal combining with oxygen to form something else. Specifically, we call that new compound an oxide. Some oxides, like water, are good. Some aren’t.
Iron combined with oxygen forms iron oxide. You know it as rust. You don’t need me to tell you this happens when iron gets wet. Iron oxide is not as strong as iron. Something made of iron oxide will break quickly. Iron oxide flakes off, meaning that it can overtake something made of iron very quickly.
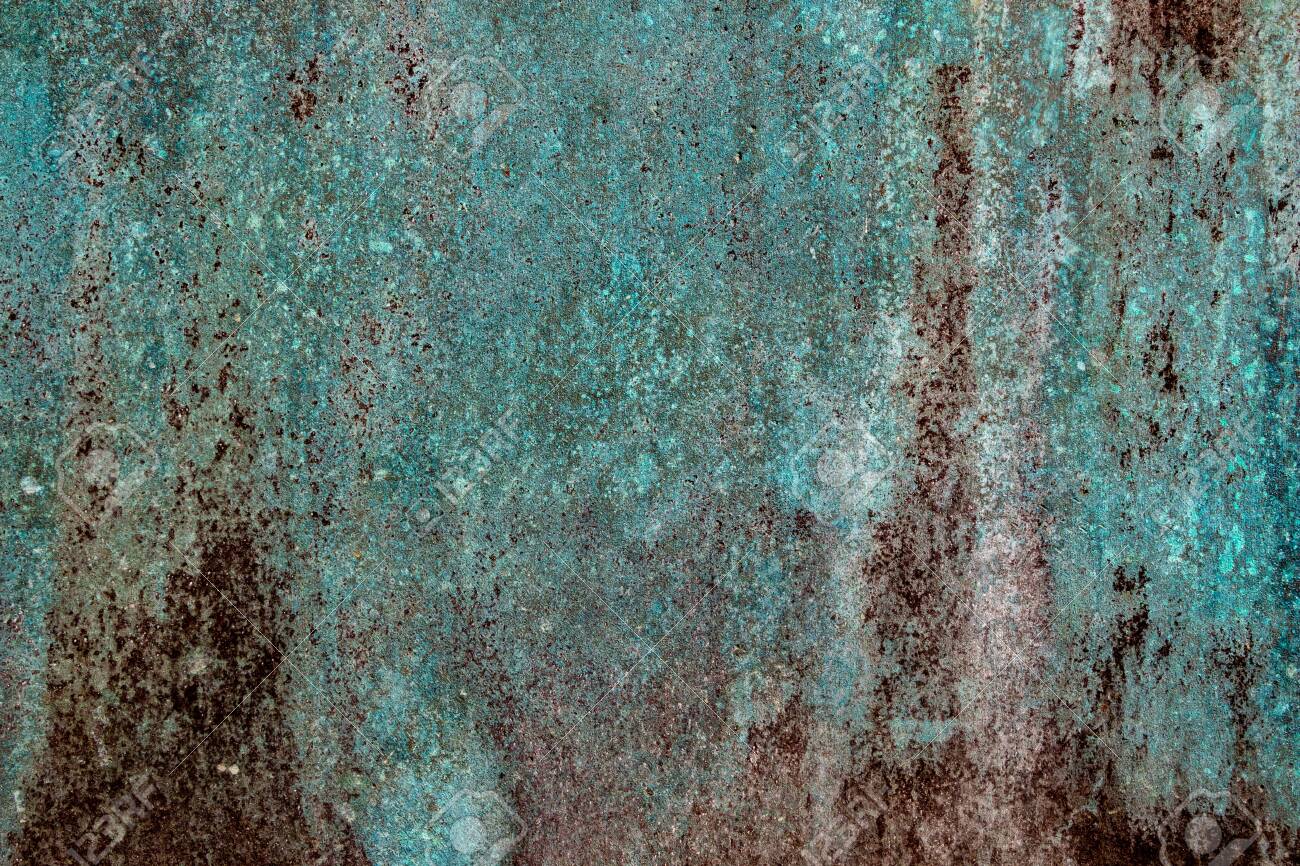
Copper also oxidizes. When it does it forms this bluish-green “patina.” This is something that architectural types really like, but it’s bad for wires. Unlike iron oxide, copper oxide doesn’t flake off. Instead it forms a protective layer which protects the copper underneath it. However, copper oxide doesn’t conduct electricity as well as pure copper so it’s the enemy of good wiring.

Aluminum oxide is the other form of oxidation that we’ll see most frequently in outdoor installations. It’s the “best” oxide of the three. Aluminum oxide is usually seen as a dull dusty black residue on an antenna. Like copper oxide it protects the aluminum underneath it. However, aluminum oxide conducts electricity pretty much as well as bare aluminum. This means it can (and should) be left in place. Its protective quality will make your antenna last longer.
What can be done to protect from oxidation?
Oxidation is going to happen, period. It’s more common in wet climates and the most common near oceans. Water makes oxidation happen faster and salty water makes it happen the fastest. The most common way to protect against oxidation is to paint a surface. That’s not always an option with outdoor antennas and equipment because the paint could make the antenna less efficient. However you will see that masts and mounts are often painted to protect against oxidation. For cables and connectors, we recommend weather boots which will keep moisture away from the inner parts of the cable.
As Neil Young said half a century ago, rust never sleeps. In some cases you’re just going to have to deal with it when it happens. But at least now you have some idea what it is and what makes it so bad.





